Breaking into Regulatory Writing with a Scientific Background: A Practical Guide for Life Science Professionals
September 17th, 2025 by Keerthi Kamagoni
The pharmaceutical and biotech industries are flourishing, and with this growth comes an increasing demand for regulatory and medical writers. These professionals play a crucial role in preparing documents that ensure compliance with regulatory authorities, such as the FDA and EMA. For scientists and life science professionals, regulatory writing offers a rewarding career that combines scientific expertise with communication skills. If you’re considering a transition, here’s a practical guide to help you break into this field.
What is a Regulatory Writer?
- Regulatory writing is a specialized and essential skill in the pharmaceutical industry. It bridges the gap between science and regulatory compliance.
- Regulatory writing involves creating documents that are submitted to regulatory authorities to gain approval for drugs, biologics, or medical devices.
- Regulatory writers, working closely with researchers, scientists, and subject matter experts, are tasked with the challenging responsibility of compiling data, analyzing scientific information, and translating complex concepts into clear and concise language that is compliant with stringent guidelines.
- The CTD structure is the backbone of most regulatory submissions worldwide. Regulatory writers are often responsible for drafting and reviewing documents in Modules 2–5.
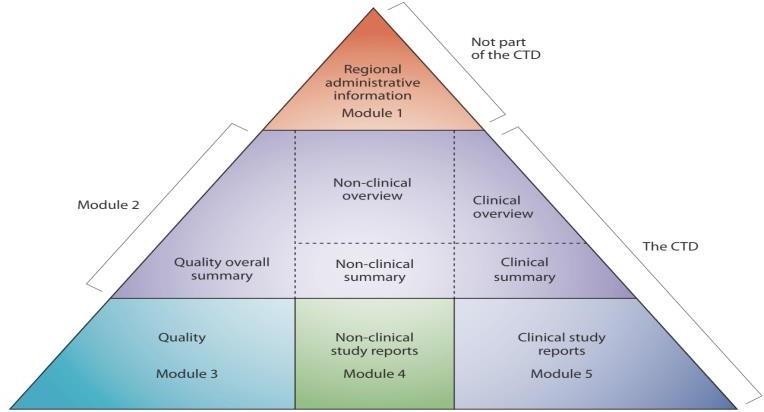
Brief outline of CTD modules:
Module 1- Regional administrative Information (Not part of the CTD)
This module should contain documents specific to each region; for example, application forms or the proposed label for use in the region.
Module 2- Common technical document summaries
It provides an overall summary of the ‘quality’ information, as well as the Non-Clinical Overview and the Clinical Overview, the Non-Clinical Written Summaries, and the Clinical Summary.
Module 3- Quality
This module presents the chemistry, manufacturing, and controls reports for the product included in the registration dossier. Full details of both drug substance and drug product are included in this module as per ICH and M4Q guidelines.
Module 4- Non-Clinical study report
The Nonclinical Study Reports should be presented in the order described in the guidance M4S.
Module 5- Clinical Study Report
The clinical study reports should be presented in the guidance M4E. It provides the clinical evidence for the safety, efficacy, and risk–benefit profile of the drug in humans.
- Regulatory writers also provide leadership, project management, collaboration, and diplomacy to the work environment, which makes them indispensable team members.
Why Scientists Make Ideal Candidates?
Scientists and life science professionals are naturally positioned to excel in regulatory writing. Your experience analyzing experimental data, interpreting results, and documenting research aligns closely with the skills required in regulatory writing.
Key transferable skills include:
- Attention to detail: Ensuring accuracy in data presentation and compliance with guidelines.
- Critical thinking: Evaluating study results and identifying inconsistencies.
- Data interpretation and synthesis: Summarizing complex scientific findings in clear, concise language.
These skills, combined with an understanding of regulatory processes, make scientists highly desirable candidates for regulatory writing roles.
Core areas to strengthen
- Study ICH Guidelines and Learn about FDA, EMA, and PMDA submission requirements.
- Regulatory writing is not creative writing; it's structured, precise, and compliance-driven.
- Understand the documents like CMC sections, Clinical study Reports, Investigational brochures, protocols, and labelling documents.
- Practice editing, reviewing the data, and ensuring consistency between tables, text, and figures.
- Learn about Good Documentation Practice (GDP)
- Build writing samples (portfolio without company tools), like creating mock CTD summaries from published studies, write summaries of journals as if they were submission-ready documents, and publish blogs.
- Regulatory writers often work with scientists, clinicians, statisticians, and regulatory affairs specialists.
- Practicing how to incorporate feedback, edit drafts, and maintain version consistency is a big plus.
Conclusion
Regulatory medical writing is a specialized discipline that plays a pivotal role in drug development and regulatory submissions. It requires a combination of scientific expertise, writing proficiency, and adherence to regulatory requirements. Transitioning into regulatory writing is not only possible but highly rewarding for scientists. By leveraging your background, acquiring focused training, and building experience, you can make a smooth shift into a field that bridges science, communication, and patient impact. Your scientific expertise is already your strongest asset now.
It’s about presenting it in a way that aligns with regulatory needs. With persistence and the right strategy, regulatory writing can be the career move that redefines your professional journey.
References
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The Common Technical Document (CTD). Available at: https://www.ich.org/page/ctd.
- U.S. Food and Drug Administration (FDA). Drugs: Guidance, Compliance & Regulatory Information. Available at: https://www.fda.gov/drugs/guidance-compliance-regulatory- information.
- European Medicines Agency (EMA). European Public Assessment Reports (EPARs). Available at: https://www.ema.europa.eu/en/medicines.
http://www.ema.europa.eu/en/medicines
Bio: Keerthi Kamagoni is a life sciences professional with a background in formulation R&D and regulatory affairs. She has hands-on experience with CTD structure, global regulatory requirements, and medical writing. Currently, she is expanding her expertise through freelance writing, portfolio development, and volunteer contributions to platforms like Bio Careers. Keerthi is passionate about bridging science and communication to support compliance and innovation in the pharmaceutical industry.
Comments are closed.
