Rediscovering My Career in Clinical Trials Without a PhD
August 15th, 2025 by Lavanya Balasubramaniam
Human life is all about miracles and surprises, and mine is no exception. Following my post-graduation, I began my career as a Clinical Laboratory Scientist in a well reputed hospital in India. I later transitioned into Medical Transcription, which allowed me to have a good work-life balance. Due to immigration policies and my role as a mother, I chose to take an 8-year long break from my career to focus on my family. When I wished to return to the workforce and rediscover my career goals, I realized it was not as simple as it appeared.
Clinical trials, as an industry, had advanced greatly from what I knew it as. There were large technological and scientific advancements within the field, which I had to catch up on. This required lots of research, reconnecting, skill developing, perseverance, and goal setting. Today, I am a Senior Clinical Database Designer at a deep rooted CRO that focuses on building complex Clinical Trials. The path to this role was difficult, but I firmly believe that “Your work is going to fill a large part of your life, and the only way to be truly satisfied is to do what you believe is great work.” Let us navigate through my journey in rediscovering my career in Clinical Trials without a PhD.
DECODING CLINICAL RESEARCH
Clinical Research plays a vital role in ensuring medical therapies and innovative medical devices are tested and evaluated with strict regulations for human use. To understand the purpose of Clinical Trials, it is crucial to identify the operations and regulations involved in the process.
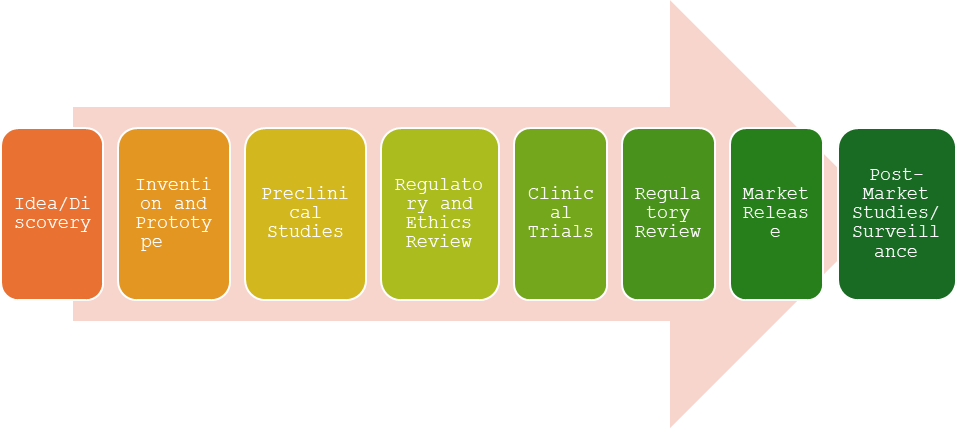
The goal is to approach this complex world in the simplest ways. Resources are widespread and require meticulous and motivated hunting. To make it easier for my future Clinical Trial specialists, I have cited below the most helpful resources that assisted me in my journey.
- The MRCT CENTER of BRIGHAM and WOMENS HOSPITAL and HARVARD (https://mrctcenter.org/)
- The Association of Clinical Research Professionals (ACRP) – A non-profit organization dedicated for clinical research professionals (https://acrpnet.org)
- National Institutes of Health – Introduction to the Principles and Practice of Clinical Research is a free 6-month certificate course offered by NIH, which effectively introduces the world of Clinical Trials to all beginners. (https://ocreco.od.nih.gov/courses/ippcr.html)
- National Institute of Drug Abuse – Offers a free Human Research Training Program.
- CITI Program – Offers GCP (Good Clinical Practices) training.
- Coursera – Offers a practical training on REDCap (Data management tool) Data Management of Clinical Research course by Vanderbilt University
- Viares Academy of Clinical Research
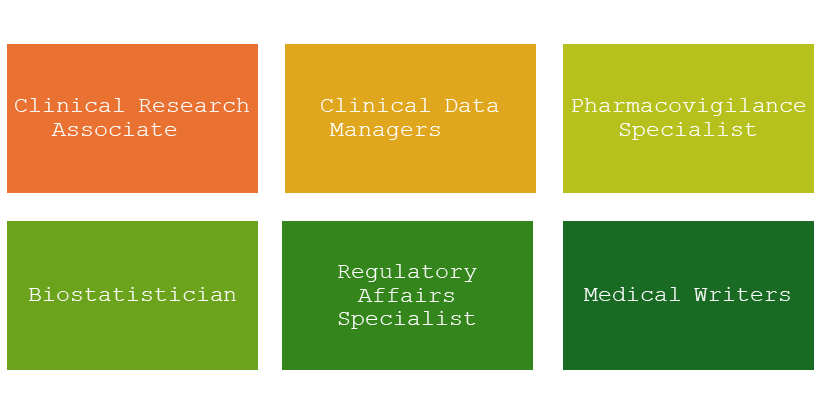
KEY STAKEHOLDERS IN THE CLINICAL TRIALS
The Clinical Trial industry has multitude of opportunities for life science graduates to create a meaningful footprint in drug development and medical device innovations. People from various life science disciplines including, but not limiting to biomedical sciences, pharmaceutical majors, biotechnology, biostatistics can embark their journey as Clinical Trial Assistant, Clinical Data Associate, Safety Associate, Regulatory Coordinator, Clinical Programmers and Medical writers. Below listed are some of the pivotal roles in the clinical trial industry:
MY CLINICAL RESEARCH JOURNEY
An important milestone in my clinical research journey was a certification in Data Management from Coursera. This program introduced me to REDCap electronic data capture system developed by Vanderbilt University, Tennessee. This course enabled me to gain a comprehensive understanding of clinical data management and regulatory framework structure emphasizing data integrity and quality.
“Medical Research involving human subjects may only be conducted if the importance of the objective outweighs the inherent risks and burdens to the research subjects” (Article 21 – Declaration of Helsinki). Comprehensive understanding of the regulatory framework was a key factor to understand the criticality of the industry.
To broaden my understanding, I ventured into learning Pharmacovigilance basically safety monitoring. Uppsala Monitoring Centre offers series of free Pharmacovigilance courses, which does introduce you to regulations involved in prioritizing human safety during the clinical trials, based on the guiding principles of Declaration of Helsinki (Foundational document in medical research ethics).
LEARNING TO LAUNCH
I wrapped up my learning with a comprehensive Clinical Research Academy program from VIARES academy. This program deepened my understanding and added a meaningful layer in gaining expertise.
With solid foundations in place, I began exploring new opportunities, starting with setting up my LinkedIn profile and reconnecting with recruiters. The journey did have setbacks, I faced failures and rejections. But ultimately, I was thrilled to join Worldwide Clinical Trials as a Clinical Data Associate.
My first role primarily involved designing Phase I clinical trials. I had the opportunity to learn the entire data management lifecycle for a Phase I study starting from designing to database lock. This knowledge base gave me the confidence to move on to my next opportunity.
CURRENT ROLE: RESPONSIBILITIES
I moved on from Worldwide Clinical Trials to another CRO as a Technical Designer. With the support from my onboarding mentor, team members and my wonderful manager I raised to my next level in the same organization as a Senior Clinical Database Designer. I gained experience in designing complex oncology trials, which enhanced my knowledge and expertise around clinical data management exponentially. Handling client relations, project timelines and budget, technical trouble shooting, and team collaboration are the key requirements of the role.
LEARNING CONTINUES….
Acquiring knowledge is a lifelong process, adapting to the evolving technology and the development of decentralized trials post COVID is transforming the responsibilities of Clinical Data Associate to Clinical Data Analyst.
Clinical data management is reshaping to Clinical Data Science as a ripple effect of data volume and complexity. The rise of decentralized trials, real-world data and genomic data are emphasizing the need for data visualization, predicting outcomes, risk-based monitoring to extend support to precision medicine and adaptive trials.
Generative AI is rapidly growing enhancing innovation and optimization and automation of trial design, data management and regulatory process. I am stepping into my learning journey once again towards data visualization and GenAI Fundamentals to grow along with the industry.
Bio: I am a postgraduate in Applied Microbiology from India. Growing up in a culture where medicine and lifestyle often begins in the kitchen, I naturally developed an early appreciation of relation between tradition and science. This ignited my passion for life science industry with deep curiosity in healthcare and innovative research and breakthroughs. I believe curiosity is the driving force behind scientific inquiry, pushing us to seek answers about our surroundings.
Comments are closed.
